Have FEP Blue and taking Dupixent? Here's what's actually covered in 2026 and what they won’t tell you about the alternatives

If you got a letter saying Dupixent won’t be covered in 2026, you’re probably starting to look into what the other covered options are – and which ones might work for you. Let’s break it down. In this article, we’ll deep dive into the FEP Blue formulary – what’s covered and not, what it means if you switch, and where the fine print matters (like age limits, boxed warnings, and off-label gaps).
And remember – you don’t have to just switch. If Dupixent is working for you, especially if some of these alternatives don’t seem like a fit, you have the right to request an exception and get Dupixent covered.
First off - what exactly is on the FEP Blue Formulary for 2026?
Formularies are long and complicated. The FEP Blue formulary for 2026 clocks in at 175 pages – whew! Let’s skip to the important stuff.
Before we go further – this isn’t medical advice. We’re here to help you understand your formulary and what it means, and you should always speak to your doctor before making any medication adjustments or changes.
Excluded drugs - Dupixent
Here’s what the formulary says about Dupixent, and the options available.
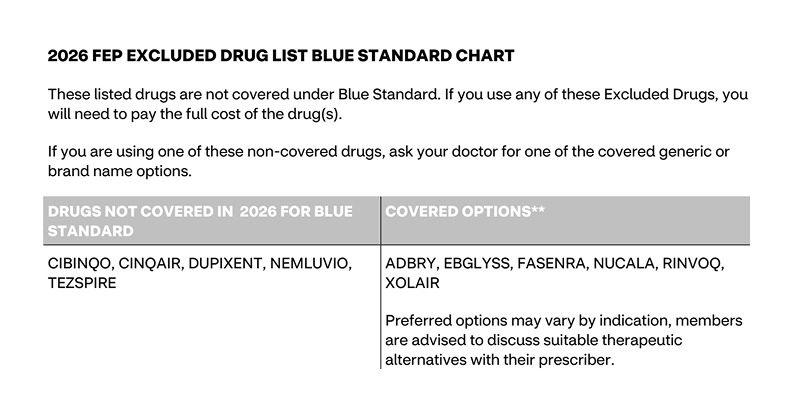
The important part here: Preferred options may vary by indication. And what’s indicated for Dupixent has a lot of variation. Dupixent is an antibody that that binds IL-4 receptor-alpha (IL-4Rα), which is the shared “docking station” for two key inflammatory messengers, IL-4 and IL-13. This means it can impact the type of inflammation that drives many different diseases – from reducing esophageal stiffness in eosinophilic esophagitis to turning down the itch signal in prurigo nodularis.
Why it’s relevant? The alternatives may work in a different way and for some indications, not at all. For example, Fasenra might be a good substitute for Dupixent for a patient with eosinophilic asthma as it can similarly reduce flares, but it wouldn’t be effective for someone with eczema – Fasenra doesn’t work on the pathways related to itch, skin barrier, or other inflammatory eczema symptoms.
What’s covered for which indication?
Don’t worry – you don’t have to dig through 175 pages of a formulary PDF to find out what you can take for what. Here are which alternatives are FDA-approved for each condition:
Not sure what these alternatives mean for you? Let’s break it down one by one – find your condition below to learn more about the specific alternatives and what to consider.
Atopic Dermatitis (Eczema)
What Dupixent alternatives are covered by FEP Blue for Asthma?
- Adbry (tralokinumab): This IL-13–only antibody is FDA-approved for patients over the age of 12 with moderate-to-severe AD.
- Ebglyss (lebrikizumab): Another IL-13–only antibody; also FDA-approved for ages 12 and up as well as over 40 kg (88 lbs).
- Rinvoq (upadacitinib): Oral JAK-1 inhibitor that is FDA-approved for AD in adults and adolescents over 12 (weight-based). Rinvoq carries boxed warnings including serious infections, malignancy, MACE, thrombosis.
How they differ from Dupixent (why it matters):
- Efficacy: Dupixent blocks IL-4 and IL-13 pathways, where Adbry/Ebglyss blocks only IL-13 – that narrower target can matter for some patients’ itch/inflammation profiles, meaning Adbry/Ebglyss may be less effective.
- Side effects: Rinvoq is a systemic immunomodulator with boxed warnings; many patients do well on it, but the risk profile and monitoring requirements are higher to avoid potentially serious complications.
Formulary flags:
- Kids under 12: There is no FDA-approved systemic alternative to Dupixent in this age group; Adbry, Ebglyss, and Rinvoq are all 12+. If your under-12 child is covered by FEP Blue and is taking Dupixent, there is no approved alternative for them to switch to. An appeal for Dupixent coverage will highlight this to show you should stay covered.
- Risk: Rinvoq’s boxed warning applies across indications; discuss risks and monitoring with your prescriber before switching. If you don’t want to risk these potential side effects by switching, you can make that argument in your appeal.
Documentation commonly required for coverage approval:
- Failure of or intolerance to topical steroids or calcineurin inhibitors (like tacrolimus)
- Baseline severity score (EASI, vIGA, POEM, or SCORAD)
- If you're currently on Dupixent: improvement shown by severity scores
- Evidence that you've been adherent to treatment
Asthma (type-2/eosinophilic or allergic)
What Dupixent alternatives are covered by FEP Blue for Asthma?
- Fasenra (benralizumab): This IL-5Rα antibody depletes eosinophils to help reduce flares in certain types of asthma. It’s FDA-approved for eosinophilic asthma for patients over the age of 6.
- Nucala (mepolizumab): Similar to Fasenra, this IL-5 antibody also lowers eosinophils. It’s FDA-approved for eosinophilic asthma eosinophilic asthma for patients over the age of 6.
- Xolair (omalizumab): An Anti-IgE antibody that’s FDA-approved for allergic asthma with positive allergy testing, in patients over the age of 6.
How they differ from Dupixent (why it matters):
- Mechanism targeting: Fasenra/Nucala work best when eosinophils drive your asthma. Xolair helps if you have allergic asthma with high IgE/sensitization. Dupixent, on the other hand, blocks IL-4/IL-13 (type-2 inflammation) and can help both allergic and eosinophilic patterns, including patients who are oral-steroid dependent. So if your asthma control on Dupixent is tied to type-2 inflammation, a switch to one of these alternatives isn’t always comparable.
Cross-condition control: If you have eczema or nasal polyps in conjunction with Asthma, Dupixent treats those too. IL-5 or anti-IgE drugs won’t help the skin or polyps, meaning you may have to try multiple drugs concurrently to address more symptoms.
Formulary flags:
- No “one-size-fits-all”: If you’re not allergic (for Xolair) or not eosinophilic (for IL-5/IL-5R drugs), these switches may be a poor fit – we can highlight this in your appeal to stay on Dupixent.
- Anaphylaxis warning (Xolair): Carries a boxed warning and may require post-injection observation. Some providers prefer Dupixent for a lower risk option.
Documentation commonly required for coverage approval:
- Eosinophil counts showing eosinophilic asthma, OR need for daily oral steroids
- History of asthma exacerbations or hospitalizations in the past year
- Failure of inhaled corticosteroids combined with long-acting bronchodilators
- If you're currently on Dupixent: fewer exacerbations or symptom improvement
Chronic Rhinosinusitis with Nasal Polyps (CRSwNP)
What Dupixent alternatives are covered by FEP Blue for Nasal Polyps?
- Nucala (mepolizumab): This IL-5 antibody reduces eosinophils, which can help with shrinking polyps, congestion, and reducing flares. It’s FDA approved for adults (18+)
- Xolair (omalizumab): Anti-IgE antibody that reduces edema and mucus to improve nasal polyp symptoms. It’s also FDA-approved for adults (18+)
How they differ from Dupixent (why it matters):
- Pathway targeting: Dupixent (IL-4/IL-13) directly targets type-2 signaling in polyp disease; Nucala focuses on eosinophils; Xolair targets allergic IgE pathways. If you’re non-allergic or your eosinophils aren’t elevated, your response to those alternatives may be weaker than to Dupixent.
- Symptom bundle: Dupixent often improves smell/taste and congestion in patients with type-2 inflammation patterns; results with IL-5/IgE agents can depend on the root cause of your symptoms.
Formulary flags:
- Allergy requirement (Xolair): Typically needs proof of allergic sensitization.
- Cross-condition control: If Dupixent is stabilizing both your polyps and comorbid eczema/asthma, a switch may help sinuses but not skin/airways the same way. This is important when weighing overall disease control.
- Anaphylaxis warning (Xolair) and phenotype mismatch (for either alternative) are legitimate concerns to document – these can help you make your case to stay on Dupixent if it’s working for you.
Documentation commonly required for coverage approval:
- Failure of at least two nasal steroid sprays and one oral steroid course
- Persistent symptoms despite 3+ months of treatment
- If you're currently on Dupixent: symptom improvement
COPD
What Dupixent alternatives are offered by FEP Blue for COPD?
- Nucala (mepolizumab): This IL-5 antibody reduces eosinophils, which can lower airway inflammation, exacerbations and steroid bursts. It’s FDA approved for adults (18+)
How it differs from Dupixent (why it matters):
- Pathway: Nucala targets IL-5 to lower eosinophils. Dupixent targets IL-4/IL-13, a different part of type-2 inflammation that’s impactful in COPD for some patients. Depending on your biomarker profile and history (ex: frequent flares despite triple inhalers), one may fit better than the other.
Formulary flags:
- Varying mechanisms: Your COPD may be driven by pathways beyond eosinophils, so some patients who respond to Dupixent might not do as well on pure IL-5 blockade like Nucala and vice versa. Without a head-to-head study, it’s hard to know at scale – and if Dupixent is working for you, you shouldn’t have to risk that uncertainty.
- Cross-condition control: If Dupixent is also controlling eczema (AD), EoE, prurigo nodularis or bullous pemphigoid, switching to Nucala won’t cover those diseases. You and your doctor may worry about flares outside the lungs.
Documentation commonly required for coverage approval:
- Eosinophil counts showing eosinophilic COPD
- Failure of standard COPD medications (inhaled steroids, bronchodilators)
- History of exacerbations despite treatment
- If you're currently on Dupixent: fewer exacerbations or symptom improvement
Chronic Hives / Chronic Spontaneous Urticaria (CSU)
What Dupixent alternatives are offered by FEP Blue for CSU?
- Xolair (omalizumab): An anti-IgE antibody that’s been a long-standing FDA-approved biologic for CSU after antihistamines fail. Approved in patients 12 and up.
How it differs from Dupixent (why it matters):
- Mechanism: Xolair targets IgE, which is the “allergic” part of CSU; Dupixent targets IL-4/IL-13, getting more at the root cause of the reaction. Some patients respond to one pathway but not the other – especially if autoimmunity plays a role.
- Logistics: Xolair dosing differs and sometimes observation is recommended. Response can be rapid or gradual, meaning it might take you some time to get back to a controlled state for your CSU,
Formulary flags:
- Boxed warning for Xolair: Xolair carries a risk of anaphylaxis – it’s important to consider, and you can argue in an appeal that you don’t want to take that risk by switching.
- Kids under 12: There is no FDA-approved systemic alternative to Dupixent in this age group as Xolair is only for 12+. If your under-12 child is covered by FEP Blue and is taking Dupixent, there is no approved alternative for them to switch to. An appeal for Dupixent coverage will highlight this to show you should stay covered.
Documentation commonly required for coverage approval:
- Persistent hives despite trying at least two different antihistamines
- Urticaria Activity Score (UAS) showing severity
- Failure of or intolerance to Xolair (if tried)
- If you're currently on Dupixent: improvement in hives and itching
Eosinophilic Esophagitis (EoE)
What Dupixent alternatives are covered by FEP Blue for EoE?
- On-label: None equivalent. Dupixent is the only FDA-approved biologic for EoE, meaning that stopping coverage is asking patients to switch to an off-label drug.
Why it matters:
- If Dupixent is working for you, you have a strong case to appeal – and you should. It doesn’t mean the off-label option won’t work for you (as always, discuss in detail with your provider), but you shouldn’t be made to switch off a stable medication that’s working for you – especially to one that hasn’t been approved for your condition.
Documentation commonly required for coverage approval:
- Diagnosis confirmed by endoscopy and biopsy showing elevated eosinophils
- Failure of proton pump inhibitor (PPI) therapy
- If you're currently on Dupixent: symptom improvement
Prurigo Nodularis
What Dupixent alternatives are covered by FEP Blue for Prurigo Nodularis?
- On-label: None equivalent. Dupixent is the only FDA-approved biologic for Prurigo Nodularis, meaning that stopping coverage is asking patients to switch to an off-label drug.
Why it matters:
- If Dupixent is working for you, you have a strong case to appeal – and you should. It doesn’t mean the off-label option won’t work for you (as always, discuss in detail with your provider), but you shouldn’t be made to switch off a stable medication that’s working for you – especially to one that hasn’t been approved for your condition.
Documentation commonly required for coverage approval:
- Diagnosis and disease severity
- Failure of phototherapy or conventional systemic treatment
- If you're currently on Dupixent: symptom improvement
Bullous Pemphigoid
What Dupixent alternatives are covered by FEP Blue for Bullous Pemphigoid?
- On-label: None equivalent. Dupixent is the only FDA-approved biologic for Bullous Pemphigoid, meaning that stopping coverage is asking patients to switch to an off-label drug. There’s evidence that some steroids and immunosuppressants can help but often have higher systemic risks in older adults – and their safety/efficacy hasn’t been approved for BP.
Why it matters:
- If Dupixent is working for you, you have a strong case to appeal – and you should. It doesn’t mean the off-label option won’t work for you (as always, discuss in detail with your provider), but you shouldn’t be made to switch off a stable medication that’s working for you – especially to one that hasn’t been approved for your condition.
Documentation commonly required for coverage approval:
- Diagnosis confirmed by biopsy
- Failure of high-potency topical steroids, oral steroids, and/or antibiotics/dapsone
- If you're currently on Dupixent: symptom improvement
So… do I have to switch from Dupixent to something else?
The short answer: No.
If the “covered alternatives” don’t match your age, diagnosis, phenotype, or safety profile – or you’re stable on Dupixent – you have a strong case to get Dupixent covered again by submitting a formulary exception.
Under federal rules, members can request exceptions to get clinically appropriate drugs covered, even if they’re not on the plan’s drug list. And if you’re in an active course of treatment, you can request an expedited decision – so you can get back on what works, fast.
How to appeal:
Claimable partners with Dupixent to make it as easy as possible for you to appeal:
- You fill out a short survey to tell us about your medical and personal history.
- We help you make the strongest case: we do the research, draft a personalized letter, include the exact FDA labels, safety language, and clinical precedents that support your diagnosis/phenotype, and get it mailed and faxed where it needs to go.
- For Dupixent appeals, our tool is free to use if you're on a commercial plan (including FEP Blue).
Bottom line
Drug lists can make switches look simple – but specifics about your condition and symptoms, age limits, boxed warnings, and off-label gaps mean the “covered alternative” isn’t always equivalent.
If Dupixent is the right fit—and especially if you’re stable on it—build the case and appeal. And if you need a hand turning your medical story and the right evidence into a winning exception, Claimable is here to help.
Be the first to know
Get the latest updates on new tools, inspiring patient stories, expert appeal tips, and more—delivered to your inbox.
You're on the list!