Resources

Latest Posts

Dr. Warris Bokhari, Co-Founder and CEO of Claimable, was named to the 2026 TIME100 Health List of the World's Most Influential Leaders in Health. The annual list celebrates innovators and pioneers working to build healthier populations around the world.
The recognition is truly meaningful. And for those of us building alongside Warris, it reflects something we've seen up close for years — steady advocacy, rigorous thinking, and a deep commitment to standing up for patients when it matters most.
We wanted to take a moment to share more about the person behind the recognition and the principles that guide his work.
A path shaped by lived experience
Warris’s work has always been personal.
He was raised in England by two parents living with long-term disabilities. His mother lived with severe rheumatoid arthritis, and his father retired when Warris was still a child because of chronic back problems. Affordable, guaranteed access to healthcare wasn’t an abstract concept in their household — it was a daily reality, directly influencing stability, opportunity, and quality of life.
That experience stayed with him. Warris trained and practiced as a physician in the UK before moving to the United States, where he later held leadership roles across major healthcare and technology organizations, including GE Healthcare, Amazon, Apple, and Anthem.
Over time, he developed a clear-eyed view of how modern U.S. healthcare actually functions — not as a system optimized for care, but as one structured around financial risk, complexity, and friction — a stark contrast to the system he experienced growing up in the U.K.
Again and again, he saw the same outcome: patients prescribed necessary care, only to face delays or denials driven more by financial incentives than medical judgment.
Why Claimable exists
Claimable was born from that inequity. Warris didn’t set out to build a healthcare company. He set out to address an escalating crisis and change what happens when patient care collides with a system built around cost control.
He brought together co-founders Alicia Graham and Zach Veigulis around a clear conviction: patients deserve real support in those moments — not more paperwork, not more waiting, and not a process designed to wear them down. From the beginning, Claimable has been built on a simple principle: patients shouldn’t have to become experts, advocates, or adversaries just to access care.
For Warris, that means not only building tools that support patients at scale, but stepping in personally when the stakes demand it.
The cases people never see
Some of the most meaningful advocacy Warris does happens out of view, supporting patients in situations where access to care is genuinely life-or-death. This includes complex organ transplant denials and advanced oncology cases, where clinical nuance, timing, and judgment matter deeply.
In many of these cases, Warris has taken the lead, navigating the medical complexity and policy reasoning that ultimately shape critical coverage decisions. Being directly involved in these moments has been both sobering and instructive, reinforcing how much responsibility comes with building in this space.
That hands-on engagement doesn’t just shape his perspective — it informs our research and development efforts, pioneering strategies in new conditions and therapies before translating them into tools within Claimable. It has pushed the boundaries of what we believe can be done at scale by combining clinical rigor with purpose-built technology. And it continually sharpens our understanding of what good judgment looks like under pressure.
“I’ve worked closely with Warris on some of the most difficult cases we’ve encountered. What stands out is his steadiness — knowing when to push, when to pause, and how to carry the weight of decisions that affect real lives.” — Zach Veigulis, Co-Founder & CAIO, Claimable
Real Patient Impact
Take the story of Keaton, a 35-year-old father who was diagnosed with Stage IV bile duct cancer confined to his liver. After an extensive multidisciplinary review, he had been fully cleared for a transplant at Houston Methodist. Despite being his only potentially curative option, the transplant was denied, effectively forcing Keaton toward palliative care.
His wife, Tori, posted online asking for help, and Warris didn’t hesitate. He stepped into one of the most complex and visible cases imaginable, not because it was easy, but because it was right.
Warris immersed himself in the clinical research, the transplant criteria, and the insurer’s policy language — and just as importantly, in Keaton’s story. He got to know the family. He understood what was on the line.
Keaton later wrote, “I honestly might not be alive today if it weren’t for Warris and the team. They are highly knowledgeable and genuinely want to help people. I would recommend them to anyone and everyone if you’re having issues with insurance or being denied a life-saving treatment like I was.”
Keaton’s story isn’t unique in Warris’s world. It’s representative of the calls he answers every day — quietly, urgently, and when the outcome matters most.
Advocate first, CEO second
Warris has always led as an advocate first: for patients, for providers, and for the integrity of medicine itself. Inside the company, that philosophy becomes culture.
He stays closely connected to the lived reality of navigating denials and keeps the urgency of this mission front and center. Whether cold-calling early provider partners, supporting families facing devastating denials, or digging into emerging research on new therapies, he sets the tone for how we operate.
Leading by example, Warris encourages us to be bold in our convictions, resourceful in our approaches, and unwavering in our integrity. That mindset has led to clear non-negotiables for Claimable: the patient story must be central; evidence must be expert-curated and accurate; patients’ rights must be defended, not sidelined; and there must always be a next step.
“I’m honored to work alongside Warris, who is a doctor by training and by creed — someone who takes ‘do no harm’ seriously in every interaction. He reminds all of us that this work is about more than overturning denials. It’s about restoring trust.” — Alicia Graham, Co-Founder & COO, Claimable
Warris’s recognition on the TIME100 Health list reflects years of difficult, often invisible work, and reinforces why Claimable exists in the first place. The lessons learned alongside individual patients continue to shape how we build — embedding empathy, rigor, and real-world insight into tools designed to support patients at scale. We’re incredibly proud of Warris for this well-deserved recognition. And we’re even more committed to the journey ahead.

Your medication worked. Your doctor prescribed it. And now your insurance says it's not covered.
Learning that your insurance plan doesn't cover your treatment is frustrating, and often leaves folks with a lot of questions. Whether you got a letter in the mail or a message from your doctor's office, you're probably wondering – what in the world is a formulary, and what do I do if my drug isn't on it?
A formulary is the list of drugs that are covered by your insurance plan. But what most people don't know is that even if your treatment isn't on the list, you can still get covered. Most plans are required to maintain a formulary exception process, and if that's denied, you have the right to appeal.
And when a formulary exception is granted, it means your insurance has to cover your treatment again – even if it's not on their official list.
Let's break down what to do if your med is "not on formulary", and how to get it covered again.
How to get a non-formulary drug covered: Quick answer
To get a non-formulary drug covered, request a formulary exception from your plan. Start by confirming why you were denied: Check your plan's formulary and get the denial reason in writing. Then, submit the exception request to your insurance. Ask your doctor for a letter of medical necessity to support your request, and clearly document any failed alternatives or other reasons why you need the exception. If your exception is denied, you have the right to appeal – and appeals supported by strong clinical evidence, legal citations, and a clear patient narrative succeed far more often than most people realize.
What Is a Formulary – and What Does "Not on Formulary" Mean?
A formulary or drug list is your insurance plan's list of approved medications. It's organized into tiers – typically ranging from low-cost generics to high-cost specialty drugs – and it determines what your plan will cover and at what cost.
When your drug is "not on formulary," it means your plan has decided not to include it on that list. When it's "non-preferred," it means they'll technically cover it, but only after you've jumped through additional hoops (usually trying cheaper alternatives first).
Here's the part most people don't realize: formularies aren't just about if a medication works. They're heavily influenced by rebate deals between insurers, pharmacy benefit managers (PBMs), and drug manufacturers. A drug can be clinically effective, widely prescribed, and still get dropped from a formulary due to behind-the-scenes business deals. The medication didn't change. The science didn't change. But the business math did.
That distinction matters, because it means a formulary exclusion is often a financial decision dressed up as a medical policy – and financial decisions can be challenged.
How Formulary Denials Happen
If you're reading this, it's probably because you got a letter, a notification, or a phone call that tells you your medication isn't covered. These notifications can come in different forms, and what you should do next depends on what you're dealing with. Find the one below that sounds like you.

You got a letter saying your medication is being removed from formulary. This is a prospective formulary change – your plan is dropping the drug on a future date. Insurers are supposed to send this 60 days in advance (though the notice is mailed 60 days ahead, that doesn't mean it arrives that early). You only get this notice if the insurer knows you're currently filling the medication. If you just switched plans or are newly prescribed the drug, you won't be notified.
You got a denial notification in your pharmacy or insurance app. A short message in your CVS, Walgreens, or insurer app telling you the claim was denied. These notifications are a starting point, but they're often frustratingly incomplete – a brief description without the full denial reason, the policy they applied, or your appeal rights. Don't assume this is the whole story.
You got a formal denial letter in the mail. This is the letter with the specific denial reason and information about your rights. It's the most complete notification – but it can take two to four weeks to arrive after the initial denial. That's weeks you could be using to prepare.
Your doctor or pharmacist told you it's not covered. Sometimes your provider checks your benefits, sees the drug isn't on formulary, and tells you they're going to switch you to something else. In this scenario, you may not receive a formal denial at all. If this happens, it's worth having a conversation with your provider about whether you want to switch, because you do have other options.
Regardless of how you found out: call your insurer and request the full documentation – the exact denial reason, the coverage policy they applied, and your appeal rights and process. Ask them to send it the fastest way possible: through your online portal, faxed to your provider who can share it with you, or emailed directly. Don't wait for paperwork to arrive on its own timeline. And don't wait for the formal denial letter to start preparing – you can begin gathering documents and building your case as soon as you know there's a problem.
The Biggest Misconception: "Not Covered" Doesn't Mean Final
The most common reaction when patients hear "not on formulary" is to assume there's nothing they can do. That it's a final decision — and that "not covered" means "can never be covered."
It's not. And this is perhaps the single most important thing to understand about the entire process.
Even many providers will tell patients "it's not covered, there's nothing we can do" – and that's simply not accurate. You have a legal right to request a formulary exception, and if that's denied, you have additional appeal rights including independent external review. Insurance companies benefit enormously from people believing that "not covered" is the end of the road. For the vast majority of denial types, it's actually the beginning.
A note on weight loss medications: If your medication is excluded specifically because your plan doesn't cover drugs for weight loss as a category, that's a plan exclusion – which is different from a formulary exclusion and significantly harder to fight. If you're in this situation, we've got a whole guide to plan exclusions here.
Formulary Exception vs. Prior Authorization: What's The Difference?
In many cases, you can't formally request an exception until there's a written denial. The PA is often what generates that denial.
What Is a Prior Authorization (PA)?
A prior authorization is when your insurer requires your doctor to request approval before a medication will be covered. Your doctor submits clinical documentation, and the insurer decides whether the drug meets the plan's coverage criteria.
A medication can be on the formulary and still require a PA. Many plans apply PA requirements to brand-name, specialty, or high-cost drugs.
If a drug is non-formulary (not on the approved drug list), coverage usually requires an exception review — and in most plans, that request is submitted through the same PA system. That's why the terms often get confused.
What is a Formulary or Medical Exception?
A formulary exception is a formal request to cover a drug that is not included on your plan's formulary. You're asking the insurer to make an exception based on medical necessity, failure of covered alternatives, lack of equivalent options, or risk of harm from switching.
How the process actually works
In many cases, you can't formally request an exception until there's a written denial. The PA is often what generates that denial.
If your current medication is removed:
Benefits are checked → A PA is required or the drug is non-formulary → A PA is submitted → The PA is denied → You request a formulary exception and/or file an appeal
If you're prescribed a new non-formulary medication:
The prescription is sent to the pharmacy → You're told it's not covered or needs a PA → A PA is submitted → The PA is denied → You request a formulary exception and/or file an appeal
If you're forced to switch and the new medication isn't working:
Your insurer requires you to switch to a covered alternative → You try the new medication → It's ineffective, causes side effects, or worsens your condition → Your provider submits a PA to return to the original medication → The PA is denied → You request a formulary exception and/or file an appeal
Why this matters: In all three situations, the prior authorization often generates the written denial that unlocks your right to appeal.
Insurance rules are layered and technical. Claimable helps you move from denial to action — so treatment decisions stay where they belong: between you and your doctor.
How to Request a Formulary Exception
Make sure you have an active denial
Before you can pursue a formulary exception, you generally need a current, documented denial.
If you've received notice that your formulary is changing on a future date, don't wait and hope it resolves itself. Ask your provider to submit a new prior authorization on the first day the change takes effect. Once the change is active, any prior approval is typically no longer valid — even if it feels like it should be.
Your provider may not automatically resubmit a PA, but they can. Just ask.
Once that new PA is denied, you have a clean, current denial to challenge.
Choose your pathway
There are two ways to pursue a formulary exception, and you can actually do both at the same time:
The provider pathway: Your doctor submits a formulary or medical exception request to your insurer, focused on clinical justification. This may include documentation showing that covered alternatives were ineffective (therapeutic failure), alternatives caused adverse effects (intolerance), and/or alternatives are unsafe due to contraindications or FDA warnings. This pathway centers on proving medical necessity.
The patient appeal pathway: You submit a formal patient appeal directly to your health plan. This is the pathway you control immediately. It allows you to go beyond clinical arguments and include how the denial personally impacts your health, life and finances and call out specific legal protections and policy inconsistencies that show your care should be covered. You can also attach your provider's medical justification.
You don't have to choose just one. Pursuing both pathways can increase your chances — think of it as "more shots on goal." If you're already researching on your own, we recommend starting a patient appeal – it puts more tools at your disposal and doesn't depend on your provider's timeline or capacity. Appeals are strongest when patients and providers work together.
A note on "formulary exception forms"
If you've been searching for a standard "formulary exception form," you're not alone. Most exception forms are designed for providers, not patients. And even when they exist, they often don't leave room to fully present your case — including clinical evidence, legal arguments, and policy support.
Don't get stuck form-hunting. You can submit a formal appeal letter directly to your plan's appeals department — or use Claimable to generate and submit the request for you. If your insurer needs additional clinical documentation, they can request it directly from your provider during the review process.
Use the right language
Here's what most guides won't tell you: the specific language you use can determine whether your request is properly categorized or quietly buried. Insurers route requests based on trigger words. If you don't explicitly ask for a "formulary exception" and state why you qualify, your request may be miscategorized as a general inquiry – which means longer timelines, less scrutiny, or it simply being ignored.
You qualify for a formulary exception under three main categories:
- Therapeutic failure – the formulary alternatives were tried and either never worked or stopped working over time. Be specific: name each medication, how long you were on it, and what happened.
- Adverse events – you experienced side effects that made the formulary alternatives intolerable. This includes reactions that led to hospitalization, allergic responses, or side effects that significantly impacted your quality of life.
- Clinical contraindication – the formulary alternatives are medically inappropriate for you. This could be due to drug interactions, an FDA black box warning for your specific situation, or a co-existing condition that makes the alternative unsafe.
State your category clearly and explicitly in your request. Don't make them guess.
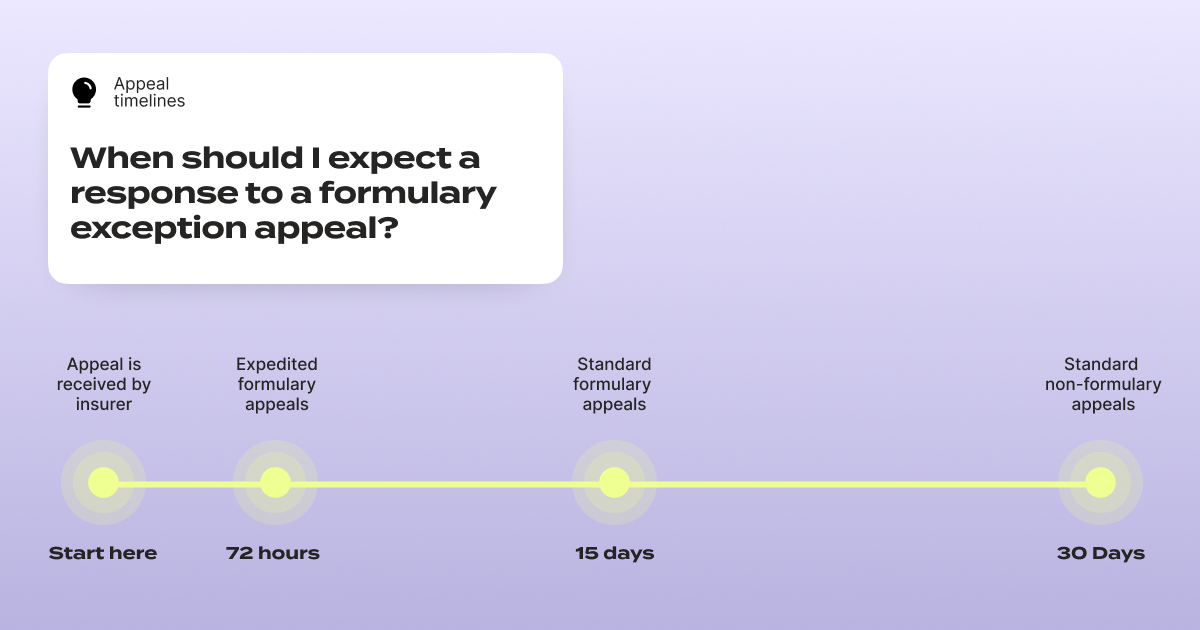
One critical timeline to know: under federal rules, insurers generally must provide expedited review within 72 hours for urgent requests and standard review within approximately 15 days for pre-service appeals – far faster than the standard 30-day review window for regular appeals.
But here's the catch: if you don't specifically request an expedited review and explain why your situation is urgent, many insurers will default to the standard timeline. If your health could seriously worsen by waiting, you have the right to request that 72-hour review. Put it in writing. At the very top of your appeal letter, write: EXPEDITED REVIEW REQUESTED (72 HOURS). Make sure it's impossible to miss.
Also double-check whether your insurer has a separate fax number or submission process for expedited appeals — they often do.
Know your rights, and state them clearly. Timelines vary by plan type, so always confirm your plan's specific rules.
Know Your Plan Type
Appeal rights and timelines can vary depending on your plan type and sponsor.
If you work for a large employer, you're likely on a self-funded plan, meaning your employer ultimately pays claims and serves as the plan fiduciary under the Employee Retirement Income Security Act (ERISA). In these cases, your appeal can reference your employer's duty to act in the best interests of employees.
Fully insured employer plans are generally subject to ERISA, the Affordable Care Act (ACA), and applicable state insurance regulations. Individual and exchange plans typically follow ACA and state rules. Federal and state employee plans, Medicare, and Medicaid each have their own appeal procedures and timelines.
Always review your plan documents — often called a Summary Plan Description (SPD), Evidence or Certificate of Coverage (EOC), plan brochure, or member handbook — to confirm the specific rules for formulary exceptions that apply to you.
What to Include in Your Formulary Exception Letter
A request that says "I need this medication" isn't enough. The ones that succeed build a structured case with specific, documented evidence. Here's what your letter should include:
The letter itself
Subject line: Request for formulary exception / appeal of non-formulary denial for [Drug Name]
Identify the denial. Your name, member ID (on your insurance card), date of denial, and the medication you were denied.
State what you're requesting. Be explicit: "I am requesting a formulary exception for [drug name], and coverage at the medically necessary level." Use the words "formulary exception." Don't leave room for miscategorization.
Explain why you need this specific drug. This is the core of your case:
- Your diagnosis and its severity, supported by test results or doctor's notes
- Why this drug is appropriate for your condition, citing clinical studies that support its effectiveness
- Why alternatives failed or are unsafe – name each one, how long you tried it, and what happened. If any alternatives carry warnings or contraindications for your situation, state that clearly.
- If you're stable on the drug: explain the improvement you've experienced and why switching creates risk – relapse, ER visits, loss of function, need for additional treatments. Spell out the real-world consequences rather than keeping it abstract.
Add legal and policy support. Reference applicable laws and protections – many states have laws against non-medical switching, and federal protections may apply depending on your plan type. If you're currently taking the medication and losing coverage could cause a gap in care, note this clearly and mark your request as "URGENT: Expedited review requested" to invoke the 72-hour review timeline.
Close with your ask and a list of supporting documents included.
And if you need help putting all of this together – that's where Claimable comes in. You answer some questions about the denial, your medical history, and personal story, and we get to work researching all the right studies, laws, and other evidence you need to build a strong appeal. Then, we fax and mail it for you. Our job is to translate your experience into a lawyer-level appeal letter, and give you the best possible chance of getting that exception approved.

The supporting documents (include as many as you have)
- Your denial documentation (notice letter, denial letter, portal screenshot or app screenshot)
- A Letter of Medical Necessity or the Medical Exception Form from your doctor
- A clear list of previously tried alternatives (drug name, dates, outcome, side effects)
- Relevant clinical notes from your medical records
- Any clinical studies supporting your medication for your condition
- A copy of your plan's rules, called a Summary Plan Description (SPD), Evidence or Certificate of Coverage (EOC), plan brochure, or member handbook
Where to find your clinical documentation: Your provider's patient portal is your best starting point (e.g., My Chart). Look for:
- Your medication list – showing what you've tried and why you stopped each one
- Your allergy list – documenting adverse reactions to specific drugs
- Visit notes from appointments where you and your provider discussed treatment decisions
If you can't find what you need in your portal, ask your provider directly for the clinical notes that document your treatment history – specifically the notes showing why alternatives failed or aren't appropriate for you.
Getting the Letter of Medical Necessity: If your provider is busy (and they always are), send them a template and specific talking points (we have one available here). Follow up – a single email that goes unanswered isn't enough when your coverage is on the line.
Common Mistakes That Waste Time or Hurt Your Request
Trying to resolve things by phone. Calling to check on the status of your request? Good to do, and can actually help – insurers have been known to claim they never received something until you provide tracking details (and then suddenly, they find it!). But don't try to appeal or negotiate a coverage decision over the phone. You don't want a low-level phone representative making decisions about your care. You want a written record, a formal process, and a qualified reviewer examining your evidence. Get everything in writing, ask them to send documentation of anything you discuss over the phone, and confirm everything they tell you in writing.
Filing a complaint with the wrong regulator. Many patients spend weeks drafting a complaint to their state Department of Insurance – only to learn that their plan is regulated at the federal level, where the state DOI has no jurisdiction. The majority of employer-sponsored plans are governed by federal law (ERISA), not state law. Before you spend time on a regulatory complaint, verify who actually regulates your plan. Your denial letter should include this information, or you can call your insurer and ask specifically: "Who handles external appeals for my plan?"
Not asserting your timeline rights. As mentioned above, formulary exceptions have faster review requirements than standard appeals. If you don't explicitly cite these timelines in your request, insurers have little incentive to prioritize it.
If Your Formulary Is Changing, Here's How to Prepare
If you've received notice that your medication is being removed from the formulary on a future date, don't wait for that date to arrive to take action.
- Get the longest supply you can now. If you're eligible for a 90-day fill, request it before the change takes effect. This gives you a buffer while you work through the exception and appeal process.
- Request a continuity of care exception. You can request a continuity of care exception to maintain coverage while your appeal is pending. Whether it is granted depends on your plan's rules, but it is absolutely worth asking.
- Have your provider file a new prior authorization on the first day the change takes effect. Your existing PA is effectively expired on the date the formulary change goes into effect, even though it shouldn't be. Your provider may not automatically resubmit a PA — but they can. Just ask. Once that new PA is denied, you have an active, current denial to appeal.
- Prepare your documentation in advance. Gather your clinical records, research the clinical evidence for your medication (or use Claimable to do the heavy lifting for you), and draft your personal statement. You don't want to be scrambling after you've been denied – you want to be ready to file immediately.
Don't Wait for the Denial Letter: Start Taking Action Immediately
You don't need to wait for the formal denial letter in the mail to start building your case. As soon as you know there's a problem – whether it's an app notification, a call from your pharmacist, or your doctor telling you they're switching your medication – make two phone calls.
Call your provider's office. Tell them you've been denied and you plan to challenge it. Ask for copies of the clinical notes that support your need for this medication – your treatment history, documentation of failed alternatives, and any relevant test results. Ask them to send it as quickly as possible.
Call your insurer. Request all documentation used to make the decision. Your denial letter (when it arrives) will likely include language stating you can request this – but you have to ask. Request:
- Clinical review notes
- Internal medical policies applied to your case
- Guidelines, criteria, or standards they relied on
- The name, credentials, and specialty of the reviewer
- Documentation of any automated systems or algorithms involved in the decision
Also file a separate claim file request – a formal request for your complete case file. This can take up to 30 days to fulfill (and insurers often don't comply unless you follow up), so getting it started immediately is smart. Consider sending it as a standalone request rather than bundling it with your appeal, since it may go to a different department.
Submit Your Appeal and Follow Up
Where to send it
Start with your denial letter or portal notice – it usually lists the appeals address, fax number, or portal upload path. If you don't see it, call the member services number on your insurance card and ask: "Where do I submit a member/patient appeal for a non-formulary denial?"
Some plans allow you to submit appeals through your online portal, which gives you a digital confirmation. If you fax, save the transmission receipt. If you mail, use certified mail with tracking.
When to follow up
If your appeal was faxed and the situation is urgent, call the next day to confirm they received it. If they say they don't have it, provide your fax confirmation details – they often "find" it once you can prove it was sent.
If your appeal was mailed, allow two to four weeks for delivery and processing. Once tracking shows it's delivered, start calling to confirm it's been logged and assigned for review.
Keep a simple log of every interaction: date, time, who you spoke with, what they said, and any reference numbers. This paper trail matters if you need to escalate.
What to Do If Your Formulary Exception Is Denied
A denied formulary exception is not the end. Your appeal rights include multiple levels of review, each with stronger protections – you can (and should!) keep fighting.
Request a second internal appeal. Your first step is a second internal appeal where a different reviewer – one who wasn't involved in the original decision – examines your case. Take a look at why they denied the request, add any additional evidence to support your case, and resubmit your appeal with REQUEST FOR SECOND INTERNAL REVIEW right at the top.
Escalate to external review. If your internal appeal is denied, you have the right to an independent external review – a decision made by a reviewer completely outside your insurance company. This is one of the strongest patient protections in the system, and insurers are bound by external review decisions.
Real Examples: Formulary Changes Happening Right Now
CVS Caremark dropping Zepbound for Wegovy
CVS Health announced that starting July 2025, Caremark would prioritize Wegovy on its standard formularies and drop Zepbound – tied to a partnership with Wegovy's manufacturer, Novo Nordisk. Patients who were stable on Zepbound were suddenly told they'd need to switch, regardless of how well the medication was working for them.
If you're in this situation, the playbook is exactly what we've described above: secure an active denial (via new PA), then submit a patient appeal showing why the forced switch isn't appropriate for you – including your treatment history, failed alternatives, and the real-world consequences of switching.
BCBS FEP Dupixent Formulary Changes
In November, BCBS FEP Blue announced that Dupixent would no longer be on their formulary. Some FEP Blue plans use a closed formulary, meaning if Dupixent isn't on the list, you pay the full cost unless you win an exception. Dupixent is a popular drug used for a wide range of conditions like atopic dermatitis (eczema), nasal polyps, asthma and COPD, and many have been impacted by this coverage change – even those who were stable and responding well to treatment.
This isn't limited to FEP. BCBS Dupixent prior authorization requirements and formulary placement vary by state and plan – what's covered under BCBS Illinois Dupixent policies may differ from BCBS Alabama Dupixent coverage. If you've been denied, check your specific plan's formulary and denial reason before assuming another BCBS member's experience applies to you.
When the plan is this strict, your appeal packet needs to be especially tight: an active denial, a clear formulary exception request using the right language, a strong Letter of Medical Necessity, documented failure history, and – if you're stable on the drug – a clear argument for why forcing a switch is medically inappropriate. Especially for conditions like EoE, bullous pemphigoid, and prurigo nodularis, for which Dupixent is the only FDA-approved treatment, the argument for getting a formulary exception is clear and powerful.
How Claimable Can Help
If all of this seems like a lot, that's because it is. Insurers intentionally make the process tough to navigate, so you're more likely to just switch when facing a formulary change. But your treatment should be up to you and your doctor – not up to a rebate deal your insurer made.
We're here to help. Claimable builds customized, evidence-backed appeal letters that combine your personal health story with clinical research, policy analysis, and legal leverage – the three pillars that make appeals successful. This isn't a template or a generic form letter – every appeal is built specifically for your situation, your medication, and your insurer.
Claimable is free for many medications and situations, and otherwise costs just $39.95 + shipping. It's a fraction of the cost of a lawyer, and most cases resolve in under 10 days. We're here to help you navigate next steps. If you've hit a wall with a formulary denial, start your appeal here.
Frequently Asked Questions
Can insurance change my formulary mid-year?
Yes. While most formulary changes happen at the start of a new plan year, insurers can make changes mid-year – including removing drugs or moving them to higher tiers. These can happen at any time but are most common on 1/1 and 7/1. They're required to notify affected patients (typically 60 days in advance), but the notification process isn't always reliable. If you suspect a mid-year change, check your plan's current formulary directly on their website.
Can insurance change my formulary without notification?
They're required to notify you if you're currently on the affected medication. However, if you recently switched plans, changed your coverage level, or are newly prescribed the drug, you likely won't receive advance notice. The notification requirement only applies to patients the insurer already knows are filling that medication.
What is a formulary exception form?
Many formulary exception forms are designed for provider submissions – not patients. If you can't find a patient-specific form (which is common), you can submit a written appeal letter with the required information to your plan's appeals department. Plans are required to accept written appeals even without a standardized form. You can also use Claimable to generate and submit your request.
What is the difference between a formulary exception and a prior authorization?
A prior authorization (PA) is a coverage review required before certain medications will be approved — even if they're on the formulary. A formulary exception asks the plan to cover a drug that isn't on its approved drug list (or to override standard formulary rules). Depending on your situation, you may need to go through one or both processes — and they often happen in sequence, which is why they're easy to confuse.
How long does a formulary exception review take?
Federal rules generally require expedited review within 72 hours for urgent requests and standard review within about 15 days for pre-service appeals — often faster than the typical 30-day window for standard post-service appeals. However, timelines vary by plan type, so always confirm your plan's specific rules and explicitly request expedited review if your situation is urgent.
What if my provider says there's nothing they can do?
This is one of the most common – and most incorrect – things patients hear. Your provider may not be familiar with the formulary exception process or may assume that "not covered" means "not appealable." It doesn't. You have legal rights to challenge formulary decisions regardless of what your provider tells you. Consider sharing resources about the exception process with your provider, or explore your appeal options independently.
Do I need a lawyer to appeal a formulary exception denial?
No. While lawyers can help with complex cases, most formulary exception appeals can be handled effectively without one. What you need is the right evidence, the right language, and knowledge of your rights. Tools like Claimable are specifically designed to help patients build strong, evidence-backed appeals without the cost of legal representation.
If you’ve been prescribed Zepbound for obstructive sleep apnea (OSA), you’re probably asking two questions: 1. Will my insurance cover it? and 2. What do I do if coverage is denied?
You’re not alone – many insurers are dropping or restricting coverage for GLP-1s, so insurance denying Zepbound for sleep apnea is a common problem. The good news is that denials in this case are worth appealing – and many people can get coverage back.
Zepbound (tirzepatide) is FDA-approved to treat moderate-to-severe OSA in adults with obesity, used along with a reduced-calorie diet and increased physical activity. In fact, it’s the only GLP-1 that’s approved to treat OSA. That means that even if your plan limits coverage of GLP-1s for weight loss, you can still get Zepbound covered for sleep apnea.
Quick answers: Zepbound + sleep apnea insurance
Q: Does insurance cover Zepbound for sleep apnea?
A: Some insurance plans cover Zepbound for sleep apnea, but coverage usually requires prior authorization. Your provider may need to submit documentation including your sleep study results, diagnosis, and proof that you meet obesity or BMI criteria. If insurance denies your request, don’t stop there. Many people are able to win coverage by submitting an appeal.
Q: What do I do if insurance denies Zepbound for sleep apnea?
A: If insurance denies Zepbound for sleep apnea, start by getting the denial letter and noting the exact reason for the denial (you may see language like “not medically necessary” or “not on formulary”). Then submit an appeal that includes your sleep study results, records of your diagnosis, obesity/BMI details, and (optional but recommended) a letter of medical necessity from your doctor. If needed, request a second review of your appeal or escalate to independent review.
If you get denied, save this quick overview of the process.
- Save the denial letter.
- Identify the reason you were denied.
- Gather documentation to support your case.
- Submit the right next move based on your denial reason.
- Escalate to a second or external review if needed.
Not sure why you were denied or which next step is right for you? Use Claimable’s easy tool to guide you step-by-step through the appeals process.
Insurance denied Zepbound for sleep apnea? Here’s what to do (Step by Step)
Step 1 — Read the denial reason and appeal instructions
Before you jump into action, find these things in your denial letter or portal message.
- The reason you were denied. Look in your letter for language like “Why your request was denied”.
- Appeal instructions. Your denial letter will provide information about how to appeal and where to send your request (fax or mail).
- Note: You might get a message in your portal before the formal denial letter comes in the mail. You don’t have to wait for the denial letter to come in order to appeal – log into your insurer’s member website and search for appeal department details.
Step 2 — Identify why you were denied
Most denials fall into one of these buckets. Look for language like one of these in your denial letter under “why your request was denied”.
When it comes to Zepbound for sleep apnea, all of these denial reasons can be challenged and you can get coverage back. It’s just about identifying the right steps to take.
- Prior authorization incomplete: The PA your doctor submitted may have missing fields or missing attachments.
- Not medically necessary: Your plan says you don’t meet their criteria to be covered for Zepbound.
- Not on formulary: This isn’t a medication included in your plan’s list of covered drugs. They’ll want you to try an alternative.
- Not a covered benefit: Your plan excludes weight loss medications and isn’t recognizing sleep apnea as the primary diagnosis for Zepbound.
- Step therapy / alternative required: They want you to try something different before they’ll approve coverage for Zepbound.
Step 3 — Choose the right next action
- If it’s missing info → ask your doctor to correct and resubmit the PA.
- If it’s criteria/medical necessity → Make sure you meet the criteria, then file an appeal. Getting a letter of medical necessity from your doctor can help here. If your insurer is requiring unreasonable criteria that doesn’t match the current FDA or clinical guidelines, Claimable can help you make that case.
- If it’s formulary → Appeal and request a formulary exception. Since Zepbound is the only GLP-1 that’s approved for sleep apnea, you should qualify for a formulary exception.
- If it’s an exclusion → Ask your doctor to file a new PA only for OSA (not obesity). If you’re denied again, appeal. Exclusions are common for obesity, but not for sleep apnea. This happens when your request is mis-categorized, so you can clear things up in an appeal.
Zepbound for sleep apnea: Coverage overview
Does insurance cover Zepbound for sleep apnea? Sometimes, yes—but it’s usually not automatic.
“Coverage” typically depends on:
- whether the medication is on your plan’s formulary
- whether you meet prior authorization criteria
- whether required documentation is submitted correctly the first time
Zepbound’s OSA indication is specifically for moderate-to-severe obstructive sleep apnea in adults with obesity (with diet and activity), so you want to make sure it’s right for you.
What insurers usually require to cover Zepbound for sleep apnea
This varies by plan, but the most common things insurance wants to see to cover Zepbound for sleep apnea are below. Call your insurer or visit your member website for a full list of coverage criteria. You can see example coverage criteria from CVS Caremark here.
Sleep study + documented OSA severity
- Sleep study report (polysomnography or home sleep apnea test, as applicable)
- Documented diagnosis of OSA and severity (often based on AHI/REI)
Obesity/BMI documentation + relevant clinical history
- Current height/weight, BMI
- Problem list / relevant comorbidities (as documented in chart notes)
Provider notes that align to plan criteria
- Recent visit notes with diagnosis and treatment plan
- Any documentation the plan requires (e.g., specialist involvement, prior treatment history)
Tip: A surprising number of denials happen because the right info exists—but it isn’t included in the PA submission or isn’t easy for the reviewer to find.
Common denial reasons and what to do about them
Denials are confusing. Here’s a breakdown of the most common denial reasons, what they look like in communications from your insurance, and how to fix it. Need help determining which reason you have and what strategy to use? Use Claimable's guided appeals tool to make it easy.
1) Prior auth incomplete / missing documentation
What it looks like: “Insufficient information,” “missing documentation,” “clinical records not provided.”
Fastest fix: Ask your prescriber’s office what they submitted, then resubmit with a complete packet.
2) “Not medically necessary”
What it looks like: “Does not meet criteria,” “not medically necessary.”
Fastest fix: Appeal using your plan’s stated reason. Clearly show that you meet the missing criteria – or that the criteria your insurer uses isn’t backed up by FDA or clinical guidelines (Claimable can help with this).
3) Not on formulary
What it looks like: “Not covered,” “non-formulary,” “preferred alternatives required.”
Fastest fix: Appeal, requesting a formulary exception. Include details about why alternatives aren’t suitable for you based on your condition or medications you’ve tried and failed in the past.
4) Benefit exclusion / “weight loss only”
What it looks like: “Plan excludes weight-loss medications,” “not a covered benefit”
Fastest fix: This is where the OSA indication matters. Zepbound is FDA-approved for moderate-to-severe OSA in adults with obesity – so an exclusion for weight-loss medication shouldn’t apply here. Appeal to make the case.
5) Step therapy / alternative requirement
What it looks like: “Must try X first,” “step edit.”
Fastest fix: Include information about why alternatives aren’t appropriate in your appeal. Lean on relevant state laws here – 37 states have laws that protect patients from step therapy requirements, so you may not be required to try and fail an alternative first.
How to get insurance to cover Zepbound for sleep apnea – before you have to deal with a denial
If your doctor is considering prescribing you Zepbound for sleep apnea, get ahead of any issues by determining if you’ll covered from the start.
What to ask your insurer (script)
Call the number on your insurance card and ask:
- Is Zepbound covered for obstructive sleep apnea under my plan?
- Is it on formulary? If not, what’s the exception process?
- What are the prior authorization criteria and where is the PA form?
- Where should the PA be submitted (portal/fax)?
- What are typical timelines, and what qualifies for an expedited review?
What to ask your provider (submission checklist)
Ask your clinician’s office to confirm the PA includes:
- Sleep study report + OSA severity documentation
- BMI/obesity documentation
- A brief medical rationale tied to criteria (not generic)
- the correct diagnosis coding and chart notes attached
Common submission mistakes to avoid
- Missing the sleep study attachment
- Outdated weight/BMI documentation
- Generic notes that don’t address the plan’s stated criteria
- Incorrect submission destination (wrong portal/fax)
How to write a Zepbound for sleep apnea appeal
Insurance is complicated, and even when you get ahead of it issues can arise. But when it comes to Zepbound for sleep apnea, most people will be able to reverse a denial when they appeal – with the right argument, documentation, and clinical backing.
What to include in your appeal
Your appeal is strongest when it mirrors the denial reason:
- Quote the denial reason (one sentence)
- Respond directly with the evidence that addresses it
- Attach the supporting documents and highlight the relevant lines. Include:
- Sleep study report
- OSA diagnosis
- Clinic notes and/or letter of medical necessity
- Clinical studies that support why Zepbound is right for you
- Any relevant laws – many states have legal protections that can help fight formulary changes, step therapy, and other inappropriate denial reasons.
If it’s a formulary appeal
- Make sure you’re clearly stating that this is a formulary exception request
- Look up what the plan is offering for alternatives, and make sure that you’re clearly laying out why those are inappropriate for you
Request a second review (internal escalation)
If your first appeal is denied:
- Request a second-level internal appeal from your plan. Do this by sending another appeal and noting your request on the first page – making sure you address the reason for their denial. If your insurance has a separate mail/fax for second-level appeals, send it there.
External/independent review (when internal appeals fail)
By law, most plans are required to offer access to external review (independent review) after a final internal denial. Your denial paperwork should tell you how to request it.
If your plan still won’t cover it
If you’ve exhausted the plan’s pathways, you can still explore:
- Employer benefits escalation (HR/benefits team can sometimes clarify exceptions)
- Manufacturer resources and savings programs (where eligible)
- Legal action. There are several class-action lawsuits underway regarding inappropriate denial of Zepbound coverage for OSA patients, or you can speak with a lawyer about your individual options.
Read our full guide here to what to do when your appeal is denied.
Always avoid delaying OSA management—talk with your clinician about other treatment options while coverage is being sorted.
How Claimable helps
We get it – navigating the insurance process isn’t always easy! That’s where Claimable comes in. Use our appeals tool to:
- Identify the most likely reason you were denied
- Create an expert-backed appeal letter that includes clinical, policy, and legal evidence to make the case for coverage for your specific situation
- Automatically mail and fax to the right place
- Escalate to the next level if your first appeal is denied
Start your Zepbound sleep apnea appeal with Claimable.
FAQs
What do I do if insurance denies Zepbound for sleep apnea?
tart by getting the denial letter and noting the exact reason for the denial (you may see language like “not medically necessary” or “not on formulary”). Then submit an appeal that includes your sleep study results, records of your diagnosis, obesity/BMI details, and (optional but recommended) a letter of medical necessity from your doctor. If needed, request a second review of your appeal or escalate to independent review.
How do I appeal for Zepbound for sleep apnea?
Write a clear letter that outlines your case for coverage: Restate the denial reason, respond with the exact evidence that addresses it, and attach the documents (sleep study, chart notes, BMI). Ask your provider for a letter of medical necessity if helpful. Mail and fax to your insurer’s appeals department.
Why did insurance deny Zepbound for sleep apnea?
Common reasons include missing PA documentation, “not medically necessary,” the drug being non-formulary, benefit exclusions, or step therapy requirements. These can all be challenged with an appeal.
What does “not medically necessary” mean in a Zepbound sleep apnea denial?
It usually means the plan believes the documentation doesn’t prove you meet its criteria. Your appeal should focus on supplying the specific missing evidence and clarifying anything the reviewer may have missed.
What should I submit if my Zepbound sleep apnea prior authorization was denied?
Resubmit the PA, or appeal with a complete packet: denial letter, PA materials, sleep study, diagnosis/severity documentation, BMI/obesity documentation, and relevant clinician notes.
Can I appeal a plan exclusion denial for Zepbound for sleep apnea?
Often, yes – plans may still have an exception process. Most plan exclusions are when GLP-1s are for weight loss – a prescription for sleep apnea shouldn’t fall under that exclusion. However, it’s common for Zepbound to be initially denied because of exclusions, and patients may need to appeal to get coverage for OSA. Even when exclusions exist, your denial letter should explain appeal rights and next steps.
What is a formulary exception and when should I request one?
A formulary exception is a request for coverage when a medication isn’t on your plan’s formulary (the list of covered drugs). It’s most relevant when your denial says “not covered” or “non-formulary.”
How do I request an independent review after my appeal is denied?
After a final internal denial, you may be eligible for external review through an independent organization; your plan’s final denial should include instructions on how to request external review. Claimable recommends always exhausting your appeals through the final pathway before giving up.
Featured stories
In the news
Download a winning sample appeal
Want to see what it takes to successfully overturn a health insurance denial? Download our sample appeal to learn how we build strong, evidence-based cases that get results.

///////////////////////////////////////////////////////////////////////
//////////////////////////////////////////////////////////////////////////
////////////////////////////////////////////////////////////////////
/////////////////////////////////////////////
Each month, I endure about eight major episodes, each one leaving me exhausted, unable to concentrate, and too unwell to take part in daily life.
The frequency and unpredictability of these symptoms have isolated me socially and limited my capacity to take part in activities most people take for granted.
///////////////////////////////////////////////////////////////////////
//////////////////////////////////////////////////////////////////////////
////////////////////////////////////////////////////////////////////
/////////////////////////////////////////////
Be the first to know
Get the latest updates on new tools, inspiring patient stories, expert appeal tips, and more—delivered to your inbox.
You're on the list!

One of our core principles is to help patients protect their rights and level the playing field with their insurance company. This includes rights to multiple appeals, fair reviews, decision rationale, exceptions when needed, and adequate network access, among others. For more, read our post on patients rights.
Claimable’s AI-powered platform analyzes millions of data points from clinical research, appeal precedents, policy details, and your personal medical story to generate a customized appeals in minutes. This personalized approach sets Claimable apart, combining proprietary and public data, advanced analysis and your unique circumstances to deliver fast, affordable, and successful results.
We currently support appeals for over 85 life-changing treatments. Denial reasons may vary from medical necessity to out of network, and we even cover special situation like appealing plans that won’t count your copay assistance towards your deductible (hint: those policies were banned at the federal level in 2023). That said, we are rapidly growing our list of supported conditions, treatments and reasons. You can quickly check eligibility and ask to be notified when your interest becomes available. It helps us know where to focus next 🙂
We think about appeal times in a few ways. First, many professional advocates and experienced patients spend 15, 30 or even 100 hours building an appeal–but with Claimable, this takes minutes. We automate the process of analyzing, researching, strategizing and wordsmithing appeals. Next, there is the process of figuring out where you will send it (hint: expand your reach beyond appeal departments), then printing, mailing and/or faxing your submission. We handle that, too. Finally, there is the time it takes to get a decision. We request urgent reviews when appropriate, and typically receive standard appeal decisions within a couple weeks.
Review periods are mandated by applicable laws, from 72 hours for urgent, 7 days for experimental, 30 days for upcoming and 60 days for received services. Our goal is to get a response as fast as possible, since most of our clients are experiencing long care delays or extreme pain and suffering.
Claims are denied for a variety of reasons, many of which blur definitions. We focus on helping people challenge denials by proving care is needed and meets clinical standards, in addition to addressing specific issues like experimental treatments, network adequacy, formulary or site of care preference exceptions. We don't support denials for administrative errors or missing information, as we think those are best handled by simply resubmitting the claim in partnership with your provider. That said, many of our most rewarding successes have been cases previously though 'unwinnable', with providers and patients who fought tirelessly for months without appropriate response or resolution.
A denial letter is a formal notice from your insurance company explaining why a claim was denied and how you can appeal the decision. Sometimes the notice is included within an Explanation of Benefits. It is a legal requirements; if you didn’t receive one, contact your insurance company.
A letter of medical necessity is a statement from your doctor justifying why a specific treatment is critical to your care and/or urgently needed. You can attach it to your patient appeal to strengthen your case, especially if you are requesting an urgent appeal or need to skip standard ‘step therapy’ requirements. That said, we don’t require them and are often successful without them.
A claim file contains all the documents and communications your health plan used to decide whether to approve or deny your claim. Most health plans are legally required to share this information upon request. According to a ProPublica investigation, reviewing your claim file can help expose mistakes or misconduct by your health plan, which can make your appeal stronger.
Your insurer is required by law to give you written information about how to appeal, including the name of the company that reviewed your claim and where to send your appeal. Your health insurer may work with other companies, such as Pharmacy Benefit Managers (PBMs), Third-Party Administrators (TPAs), or Specialty Pharmacies, to manage your claims. These companies might be responsible for denying your claim and handling the appeal process on behalf of your insurer.
If you don't win your first appeal– don't give up! Many people are successful on their 2nd, 3rd or even 4th try, and future appeals are reviewed by independent entities. That said, we wrote a whole guide to understanding your options, including escalating your appeal and seeking other assistance for covering costs, forgiving debt or even seeking legal or regulatory support.
While both denial rates and appeal success rates vary widely by the type of health plan, state, and insurance company, studies have shown more than 50% of people win their appeal–and we apply strategies to boost your chances of success. Claimable has an 80% appeal success rate. The biggest denial challenge is that most people never appeal–allowing unjust denials to control their healthcare options because they are unaware of their rights or lack the support needed to fight back. No one needs to fight alone–Claimable is here to help. We know first hand that many denials are based on errors, inconsistencies or auto-decisions, and have proven strategies for fighting back against this injustice.
Let’s get you covered.




